Investor and leader in Biotechnology.
Matti Kesti is a biotechnology leader with a PhD from ETH Zurich. He has over 10 years of experience as the CTO/CSO in the biotech sector. He is a serial entrepreneur and active member in Swiss startup ecosystem. He is a husband, a father of four and a passionate mountain climber in the Swiss alps. He has both the Swiss and Finnish citizenships, and is well connected internationally.
Executive summary
Specialist in bioprinting
He has MSc in Materials Engineering and PhD in bioprinting technologies at ETH Zurich. He was the CTO of Auregen Biotherapeutics, the company that translated bioprinted ear graft cell therapy to FDA clinical trials.
Innovated many patents in bioprinting technologies and cGMP processes to enable clinical translation of bioprinting.
Worked with bioprinting industry partners to develop their printers to be suitable for cGMP production.
Led the bioprinting scientific advice and technical discussions with EMEA and FDA.


Skills
Angel investor in early-stage companies. In 2025 joined the Switzerland's largest angel investor group SICTIC as a member. In 2025 joined the ETH Zurich entrepreneurship mentor program to advice university founders and their companies.
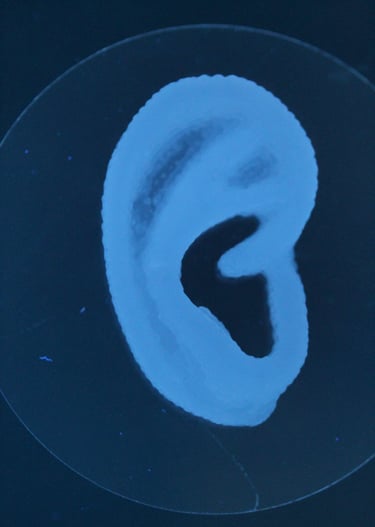
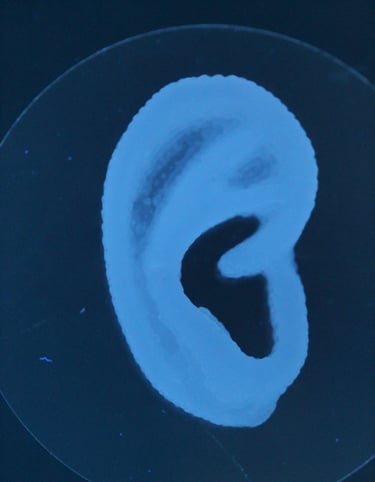
First 3D bioprinted cell therapy in clinical trials. Auregen Biotherapeutics, an ETH Zurich spin-off, was built on his PhD research to translate 3D bioprinted cartilage ear grafts. During his leadership in chemistry, manufacturing and controls (CMC) group the company translated the technology to clinical trials under FDA (NCT06072040).
Graduated ETH Zurich PhD in 2018 from Prof. Marcy Zenobi-Wong group, specializing in 3D bioprinting technologies.
Founding Auregen Biotherapeutics SA in 2016.
Moved to Switzerland 2012 for biotech research and received the Swiss citizenship 2022.
Career Highlights
Leadership and management
Board Member
Executive and cross-functional leadership
Strategic Partnerships
Investor Relations
Company formation and recruitment
CapEx budgeting and financing
Entrepreneurship
Mentoring
Program and project management
Technical
AI agents and GNN
Scientific due diligence
Biopharmaceuticals
Cell & Gene Therapies
Tissue engineering (Cartilage, Bone, MSC)
Release process and quality assurance
Technology transfer to CDMO
IP rights, licensing, portfolio management
Pre-clinical package (ISO-10993, Tox, PD, etc.)
Innovation management
Regulatory
Regulatory advocacy and strategy
CMC representative in multiple regulatory meetings (FDA, EMEA, etc.)
Manufacturing license approval
Audit readiness and audit participation
Pre-IND and IND meetings
IND module 3 author and complete IND reviewer
User testing and design history file
Professional Experience
Angel Investor member
SICTIC, Switzerland
March 2025 - Present
Member of the Swiss ICT Investor Club (SICTIC). SICTIC is the largest angel investor club in Switzerland with over 500 members.
Chief Technology Officer
Auregen Biotherapeutics, Switzerland
December 2016 - February 2025
Co-founder, CTO and board member at Auregen. Directed and developed teams across global sites (CH, DE, USA). Participated in cross-functional leadership, shareholder and board meetings. Experience in both fundraising and budget allocation, growing the company from a university spin-off to a clinical stage biotech.
Strategized and oversaw required pre-clinical development, IND enabling development studies and manufacturing process.
Build the R&D group and led the team of 10 FTEs. Responsible of the budget oversight and vendor contracts.
Invented multiple new technology patents and managed an IP portfolio with a legal team.
Led technology transfer to a CDMO and represented the client in process development and validation. Led analytical in-process and release test methods transfer to QC. Experience in aseptic manufacturing and cGMP requirements.
Represented CMC organization with global regulatory bodies (FDA, EMEA, PEI, TGA) and successfully prepared regulatory submissions and briefing documents, facilitating clinical trial approvals and venture capital investments.
Build strong supporting networks from KOLs, investors, CROs, partners and collaborators. Facilitated multiple user testing and surgical training days with KOLs and lead surgeons.
PhD Research
ETH Zurich, Department of Health, Science and Technology, Switzerland
January 2013 - December 2016
Matti performed his PhD research in Tissue Engineering and Biofabrication group of Prof. Marcy Zenobi-Wong. The Prof. Zenobi-Wong group is known for expertise in innovative 3D bioprinting methods, cutting edge materials science and deep understanding of cartilage biology. Multiple graduate PhDs have founded spin-off companies based on their research in the group.
During the PhD, Matti collaborated with multiple bioprinter providers including RegenHU and Cellink to provide feedback for further product development. Matti supervised multiple master thesis projects and collaborations from external laboratories such as AO Research Institute Davos. He was part of the organizing committee of 3D printing and bioprinting summer school at EPFL. He was a speaker and presenter in multiple biofabrication conferences and academic research meetings.
Teaching Responsibilities:
Competence on additive manufacturing of polymeric materials summer school (3D CAMP), September 5th-9th 2016, Lausanne, Switzerland
Member of the summer school organizing committee. This was the first in Switzerland additive manufacturing summer school aimed to teach doctoral students about the new technological development in flexible electronics, composite materials and Biofabrication. He was the main responsible of the Biofabrication workshop and study package.
Swiss Medical Student Convention (SMSC), November 13th-15th 2015, Fribourg, Switzerland
According to the convention theme ”SECOND HAND, how reusable is your body?” He worked as a teacher in a module: Clinical and experimental approaches for cartilage (re)engineering. Module consisted of lectures on current clinical cartilage reconstructions and a hands-on workshop in future cartilage tissue engineering approaches.
Matti developed multiple hydrogel formulations "bioinks" for extrusion based bioprinting. His materials science experience from PLA and PCL extrusion to stimuli-responsive hydrogels were basis for multiple scientific publications. Many bioinks were developed with in vitro cell interaction studies before testing the lead formulations in vivo. Novel bioprinting processes developed during the research included removable support printing with Pluronics that allowed overhanging features with cell-laden bioinks and composite reinforced printing to improve mechanical properties of large cartilage constructs such as ear cartilage grafts.
Research publications:
A versatile bioink for 3D printing of cellular scaffolds based on thermally and photo-triggered tandem gelation. Kesti Matti, Müller Michael, Becher Jana, Schnabelrauch Matthias, D'Este Matteo, Eglin David & Zenobi-Wong Marcy. (2014) Acta biomaterialia. Vol. 11.
Bioprinting Complex Cartilaginous Structures with Clinically Compliant Biomaterials. Kesti Matti, Eberhardt Christian, Pagliccia Guglielmo, Kenkel David, Boss Andreas & Zenobi-Wong Marcy. (2015) Advanced Functional Materials. Vol. 25.
Guidelines for standardization of bioprinting: a systematic study of process parameters and their effect on bioprinted structures. Matti Kesti, Philipp Fisch, Marco Pensalfini, Edoardo Mazza & Marcy Zenobi-Wong. (2016) BioNanoMaterials Vol. 17, PP. 193–204.
Biomaterial researcher
AO Research Institute Davos, Switzerland
July 2012 - December 2012
AO Foundation specializes in the treatments of trauma and disorders of the musculoskeletal system. Matti was working in musculoskeletal regeneration group under supervision of Dr. David Eglin and Dr. Mauro Alini as a polymer chemist. Research was focused on hyaluronic acid hydrogels and their use in tissue engineering scaffolds and drug delivery applications.
Biomaterial researcher Intern
Tampere University of Technology, Finland
April 2012 - June 2012
Matti was working in the biomedical engineering laboratory as an assistant. Work consisted of melt extrusion of PLA/PGA based fibers and their analysis. He analyzed polymer blending affects on melting and glass transition temperature of PLA/PGA material and modified PLA fibers used in further implant manufacturing. Performed degradation and release kinetics studies for fiber scaffolds used as clinical joint spacers in rheumatoid arthritis and in osteoarthritis (RegJoint, Scaffdex ltd.). The work was done under supervision of Professor Minna Kellomäki and Dr. Niina Ahola.
Chief Science Officer
Floatz, Switzerland
November 2025 - Present
Co-founder, CSO and board member at Floatz. Floatz is an AI first due diligence platform enabling biotech investors. Designed to perform multi-factorial scientific due diligence to assess scientific risks, freedom to operate and translatability based on all collective research experience. This comprehensive stress-test will find potential scientific flaws or issues behind each technology. We provide objective and auditable evidence to assess the scientific thesis and build data-driven conviction for the investors.
Directed and developed teams across global sites (CH, USA). Participated in cross-functional leadership, shareholder and board meetings. Worked with leadership team in fundraising, budget allegation and company strategy.
Responsible for AI agents training and optimization for biopharma searched. Leading teams to validate AI reports and improve accuracy.
Education
PhD, ETH Zurich, Switzerland 2013 - 2018
Department: Health science and technology, Institute: Institute of biomechanics
PhD thesis: Bioprinting technologies for auricular cartilage tissue engineering
MSc (Tech.), Tampere University of Technology, Finland 2012 - 2013
Department: Material Engineering
Major: Biomaterials, Minor: Industrial management
Master's thesis: Developing 3D bioprinting inks based on hydrogels (ETH Zurich)
Laboratory assistant in the Department of Biomedical Engineering (TUT).
BSc (Tech.), Tampere University of Technology, Finland 2007 - 2012
Department: Material Engineering
Major. Biomaterials, Minor: Industrial management
Bachelor's thesis: Acromioclavicular Joint Fixations (Bioretec Oy, Finland)
Professional Development
Cell & Gene Therapy Products - CMC & Quality Requirements, Fleming Training September 2022
Stability & Shelf Life of Cell & Gene Therapy Products, Fleming Training March 2023
Introduction to Generative Al, Google Cloud/Coursera January 2024
Generative AI for Business Leaders, LinkedIn June 2024
Project Management Fundamentals, Microsoft February 2025
Management Strategies Seminar Schmidhauser & Partner AG, July 2025
Board of Directors Certification Startup Board Academy, October 2025
Languages
English Full Proficiency
German Fluent
Finnish Native
Mandarin Chinese Basics
IT & Software
Microsoft Suite Excellent
AI and prompts Excellent
QA, Veeva Vault Good
3D printing, CAD Excellent
3D scanning Good


Integrity, trust and strong family